1. Background
Cellular heterogeneity is widespread, which restricts the greater application of stem cell therapy in regenerative medicine and clinical care. Single cell isolation and collection technology is a key part of solving the heterogeneity problem, and exploring non-invasive, efficient and high-throughput single cell isolation and collection technology has been increasingly emphasized by scientists.
Traditional flow cell sorting techniques require fluorescent labeling pretreatment, which is time-consuming, inefficient and affects cell function. Common microfluidic sorting techniques, such as droplet microfluidics, place individual cells inside a droplet after wrapping, but according to the Poisson distribution, the single-cell capture efficiency is not high, and the position of the microdroplet after wrapping is not fixed, and cannot be observed in real time.

The R&D team from the University of Science and Technology of China (USTC) designs a single-cell separation system based on the principle of combining real-time cell recognition and microfluidic impact printing to achieve label-free, high-efficiency, real-time recognition and high-throughput separation of single cells.
2.Research content
This single-cell separation system consists of three major components: signal control module, image processing module and printing module, as shown in Figure 2.

Under the action of the pressure pump, the cell suspension is transported from the inlet to the outlet of the microchannel, and the Thousand Eyes Wolf high-speed camera equipped with an inverted microscope focuses on the observation plane in the middle of the microchannel, captures the cell grayscale image at a speed of 2620 frames per second, and real-time background extraction, Gaussian de-noising, and threshold segmentation of the image to identify and optimize the location of 2D cells, and then sends a trigger signal to the signal control module, triggering a piezoelectric Then a trigger signal is sent to the signal control module, which triggers the piezoelectric actuator to impact the flexible film on the print chamber, causing droplets containing the recognized cells to be ejected from the nozzle onto the substrate.

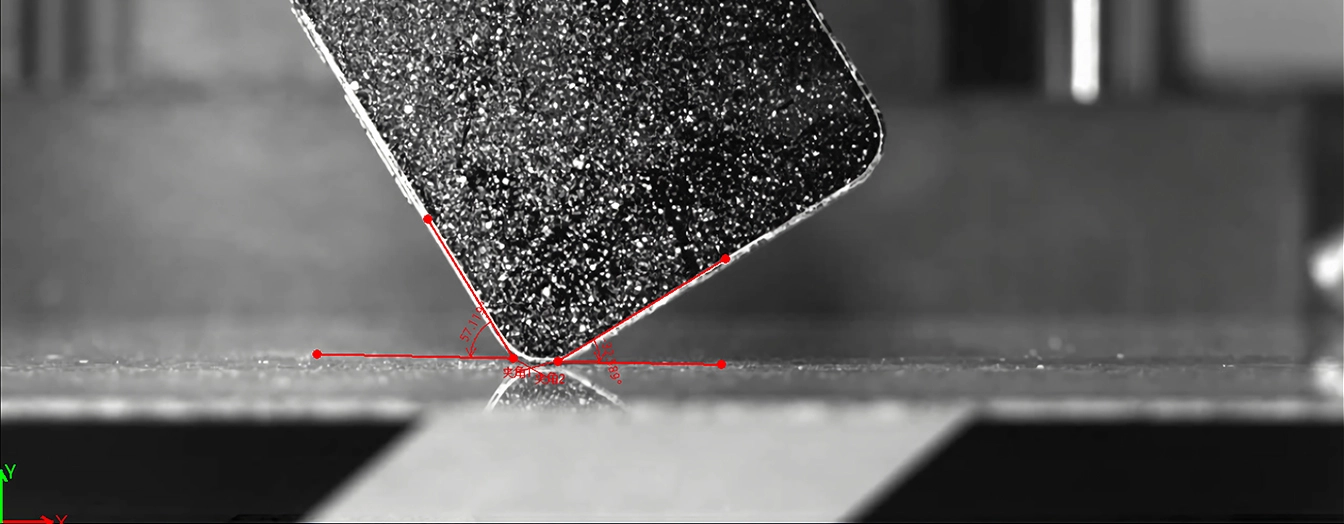
During microfluidic impact printing, affected by the hydrodynamic response, cells may move laterally and forwards during droplet ejection, which affects the reliability, and it is necessary to capture the transient images of the cell movement with a high-speed video camera under a 5× objective lens, as shown in Figs. 4 and 5, in order to explore the factors that determine the cell ejection and printing efficiency.
3.Research Conclusion
Through a series of experiments using 10 μm polystyrene microbead jetting, it was demonstrated that the single-cell separation system based on combining real-time cell recognition and microfluidic impact printing can separate single cells in a label-free manner in an efficient and high-throughput manner, with a single-cell droplet printing throughput of up to 15 Hz, and supports a one-step separation of heterogeneous single cells from cell populations with a printing efficiency of more than 95%. In the study of cellular hydrodynamic response mechanism, the position of cells in the channel crossing zone, piezoelectric actuator driving voltage, and droplet volume are the key variables affecting the printing efficiency as observed by a high-speed camera.

4.Industry Prospects
The technology will have a wide range of prospects for application in a number of biochemical fields such as emerging biological tissue printing, tumor typing, drug screening, chemical crystallization, and genetic analysis. Observation instruments high-speed cameras play an important role in the single-cell separation stage of the transient image capture of cell movement and and microfluidic impact process of fluid dynamics response mechanism research.